The Tregs develop in the thymus and require two things: TCR signaling and IL-2. The thymus expresses a very diverse set of epitopes including that from peripheral tissues such as the pancreas or prostate. The high-affinity interaction between TCR and epitope/MHC II makes proto-Treg sensitive to local IL-2, a necessary step to complete a Treg formation loop.
But what cell provides that crucial IL-2 to proto-Tregs? There hasn't been any consensus with this regard but a new paper in the Journal of Experimental Medicine from Sasha Rudensky's lab indicates that it is mature CD4+ T cells and CD25+Foxp3- CD4+ single-positive (SP) T cells that are the main source of thymic IL-2 required for Treg development.
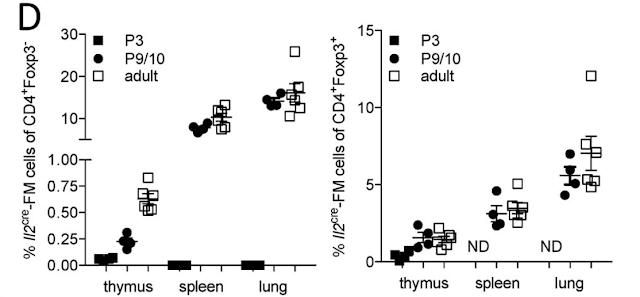
For this study, they used an IL-2 reporter mouse wherein cells expressing or having a history of the expression of IL-2 are genetically labeled and analyzed. They found that IL-2 expression was restricted to TCRbeta expressing CD4+ population.
Out of CD4+ T cells, the most IL-2 was made by mature CD4 SP and CD25+Foxp3- CD4+ T cell population. Of note, CD25+Foxp3- T cell population contains proto-Tregs.
Interestingly, the authors also detected mature Tregs with the history of IL-2 expression. It implies that bifurcation between Tregs versus IL-2 producer is a stochastic process.
As expected, TCR signaling together with IL-2 was essential for Treg formation. A "bystander" effect on Foxp3 upregulation on antigen-independent proto-Tregs (Vbeta 8- T cells) could be explained by the fact that these T cells were likely TCR activated in vivo before harvesting for ex vivo experimentation.
Based on these data, the authors suggested the following model: among mature SP CD4 T cells, a small pool produces IL-2 that in the context of high-affinity TCR/epitope interaction and CD25 upregulation promotes Foxp3+ Treg formation either autocrine or paracrine manner. Since the thymus is expressing self epitopes we can conclude that those IL-2 producing T cells are auto-reactive T cells.
1. What determines Treg, IL-2-producer or deletion pathways? All three options are open for high-affinity TCR+ CD4 SP cells.
2. Do TCR specificity overlaps between Tregs and IL-2 producers?
3. What cells provide IL-2 to Tregs in the periphery?
4. Is IL-2 delivery TCR/epitope-specific or non-specific event?
We have recently published a new model, called SPIRAL, that provides answers to these questions. The SPIRAL is based on the principle of epitope cross-reactivity.
Shared TCR epitope cross-reactivity could permit dyads of Foxp3+ regulatory and IL-2-producing T cell precursors to escape thymic purge
posted by David Usharauli