A new study in Journal of Experimental Medicine showed that allergen-sensitized pregnant mothers transfer protection against the same allergen to their offspring via milk immune complexes.
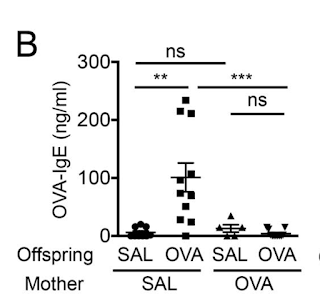
In this study the authors showed that mouse pups born to mothers sensitized to allergen were significantly protected from developing allergic response to the same antigen.
Protection in offspring was associated with the generation of antigen-specific Foxp3+ Tregs as observed in proliferation suppression assay or following short-term Treg depletion by DT (however, the authors did not analyze antigen-specificity of Tregs by tetramer staining).
Further experiments showed that mother's milk contained allergen-specific antibodies and immune complexes (IC) and breastfeeding by allergen-sensitized mother (irrespective of birth mother status) was sufficient to transfer allergen protection to offspring.
In summary, this study suggests that breastfeeding by allergen-sensitized mothers can benefit offspring by preventing development of allergic response to the same allergen. However, it is not clear how exactly the authors see this mechanism working in humans. In mice, mothers were intentionally sensitized with allergen using epicutaneous (skin) application that supposed to mimic how humans with skin barrier dysfunction get sensitized to allergens. But the authors have not tested if milk from atopic human mothers can have the same effect on their offspring. For some reason the authors tested milk from nonatopic human mothers and showed that it 'worked' when fed to mice but did not provide any explanation why healthy, nonatopic human mother milk should contain any "protection" against allergen when mothers themselves are not sensitized as experiments in mice showed they must be for a milk derived immune complexes to work. So lots of unknowns and contradictions.
posted by David Usharauli