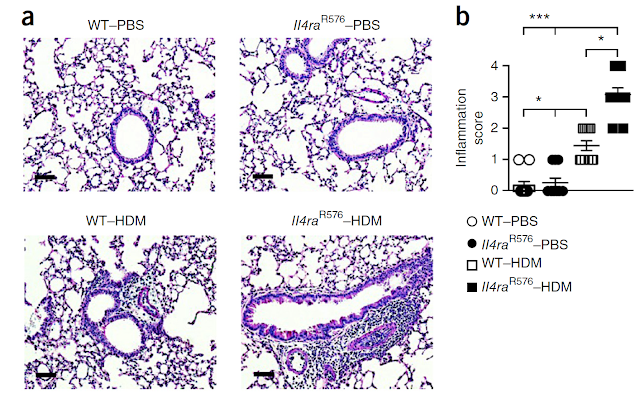
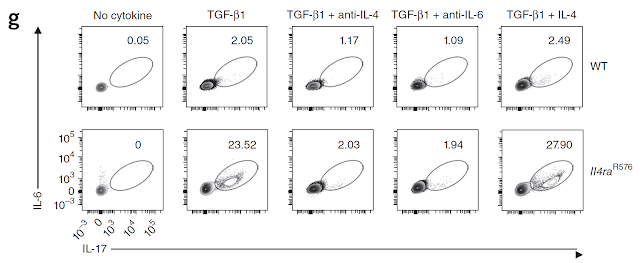
This is my first review of a paper published in new journal from Science family called Science Immunology.
This paper deals with the relationship between TCR and origin of peripheral [gut-residing] Foxp3+ regulatory T cells. Specifically, the authors have used method called somatic cell nuclear transfer (SCNT) that allowed generation a transnuclear (TN) mouse that carries a TCR cloned from gut tissue Foxp3+ Tregs.
First surprising observation was that these Foxp3+ TCR transnuclear but otherwise unmanipulated mice (called pTreg TN/RKO mice, on RAG KO base) had no Foxp3+ Tregs neither in the thymus or the peripheral lymph nodes (mLNs).
Second, the authors found that transnuclear mice instead harbored specialized IFN-γ+CD8αα+ intraepithelial lymphocyte (IEL) subset.
Second, the authors found that transnuclear mice instead harbored specialized IFN-γ+CD8αα+ intraepithelial lymphocyte (IEL) subset.
Indeed, adoptive transfer Foxp3+ TCR transnuclear T cells into WT host revealed that donor T cells could differentiate either into CD8αα+ intraepithelial lymphocyte (IEL) subset or acquire Foxp3 marker as representative of peripheral Treg subset. Of note, development of both of these subsets dependent of gut microbiota.
Finally, the authors showed that donor Foxp3 transnuclear T cells transferred into T cell-deficient hosts did not induce gut inflammation even if they were derived from scurfy background (lacking ability to express functional Foxp3 protein), implying that functionality of CD8αα+ intraepithelial lymphocyte (IEL) derived from Foxp3+ TCR T cells did not cause any tissue pathology.
In summary, this study showed that depending on circumstances TCR derived from intestine Foxp3+ Tregs can drive development of two distinct subsets with immunoregulatory role: CD8αα+ intraepithelial lymphocyte (IEL) subset or peripheral Foxp3+ Treg subset.
David Usharauli
Finally, the authors showed that donor Foxp3 transnuclear T cells transferred into T cell-deficient hosts did not induce gut inflammation even if they were derived from scurfy background (lacking ability to express functional Foxp3 protein), implying that functionality of CD8αα+ intraepithelial lymphocyte (IEL) derived from Foxp3+ TCR T cells did not cause any tissue pathology.
In summary, this study showed that depending on circumstances TCR derived from intestine Foxp3+ Tregs can drive development of two distinct subsets with immunoregulatory role: CD8αα+ intraepithelial lymphocyte (IEL) subset or peripheral Foxp3+ Treg subset.
David Usharauli