The inherited immunodeficiencies are frequently characterized with dysregulated Th2 responses, atopy, and elevated IgE levels. Mutations in Foxp3, STAT3, DOCK8, PGM3, LAT, ZAP70, or RAG result in hyper IgE phenotypes. Mechanism is unclear.
This time the scientists found that patients with another inherited immunodeficiency disorder, called Wiskott-Aldrich syndrome (WAS), also show elevated IgE signature and increased frequency of sensitization to food allergens. In mouse model, selective deficiency of WAS protein in FOXP3+ Tregs could recapitulate hyper IgE signature.
For this study the researchers analyzed "the overall burden of clinical food allergy within a cohort of 25 patients with mutations in the WAS gene" and found that individuals with WAS mutations were more likely to demonstrate serum sensitization to peanut, milk, and egg (compared to the general population).
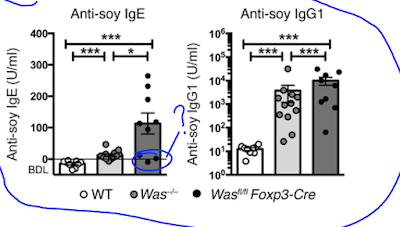
Lab mice deficient for WASp also display elevated IgE (and IgG1) antibody levels to components of their chow food (even on germ-free background).
Since WASp is expressed in different cell types, the authors tested mice selectively deficient for WASp in B cells, DCs or FOXP3+ Tregs. Out of these gene-modified mice, only Wasfl/fl Foxp3-Cre mice showed deviation to Th2 phenotype and development of IgE to chow food.
In vitro T cell stimulation confirmed that total T cells from Wasfl/fl Foxp3-Cre mice showed selective enhancement in Th2 cytokines.
In sum, these results indicate that mice with selective deficiency of WASp in FOXP3+ Tregs display excess in Th2 subsets. It is possible that absence of WASp destabilizes FOXP3+ Tregs and this somehow drives their de-differentiation into Th2-like cells (and not into Th1 or Th17, for instance).
David Usharauli
In vitro T cell stimulation confirmed that total T cells from Wasfl/fl Foxp3-Cre mice showed selective enhancement in Th2 cytokines.
In sum, these results indicate that mice with selective deficiency of WASp in FOXP3+ Tregs display excess in Th2 subsets. It is possible that absence of WASp destabilizes FOXP3+ Tregs and this somehow drives their de-differentiation into Th2-like cells (and not into Th1 or Th17, for instance).
David Usharauli