Here is another paper that highlights unsuspected health risks of commonly used food emulsifiers, carboxymethylcellulose (CMC) and polysorbate-80 (P80).
Ordinarily, mucus separates gut epithelial cells from the gut microflora. New study in Nature provided evidence suggesting that this separation of gut epithelial cells from the gut microflora is disrupted by CMC and P80, two commonly used food emulsifiers (but not by sodium sulfite, another commonly used food additive).
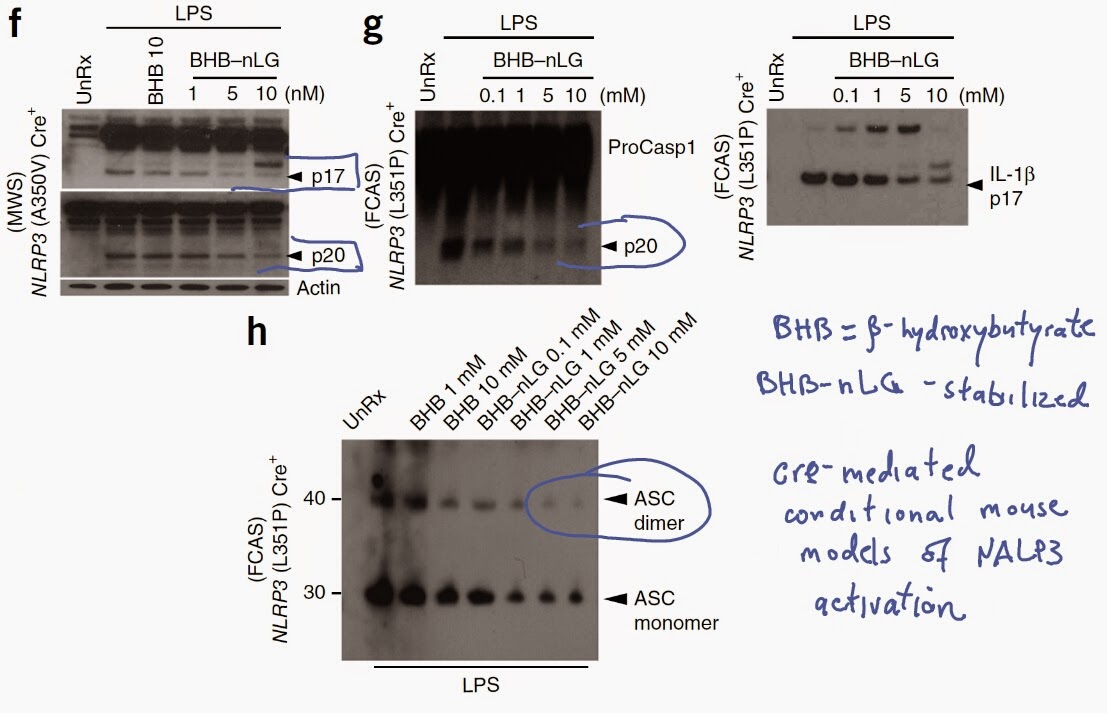
The authors showed that presence of either CMC or P80 in either drinking water or dry food pellets could promote development of colitis in susceptible mouse strain (IL-10 KO).
In addition, the authors showed that presence of either CMC or P80 in water or dry pellets could promote development of metabolic syndrome in wild-type as well as in susceptible mouse strain (TLR5 KO).
Interestingly, metabolic syndrome did not develop in germ-free mice treated with CMC or P80.
More importantly, effects of CMC or P80 on animal health were transferable to germ-free mice by fecal transplantation from treated mice, implying direct role of modified gut microflora (note, CMC or P80 did not directly modify mucus thickness in germ-free mice).
In summary, these results support the growing evidence that chemically-processed food carries long-term health risks (development of type II diabetes, metabolic syndrome, inflammatory bowel disease).
David Usharauli