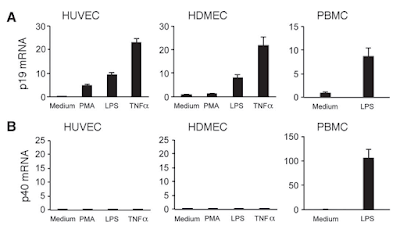
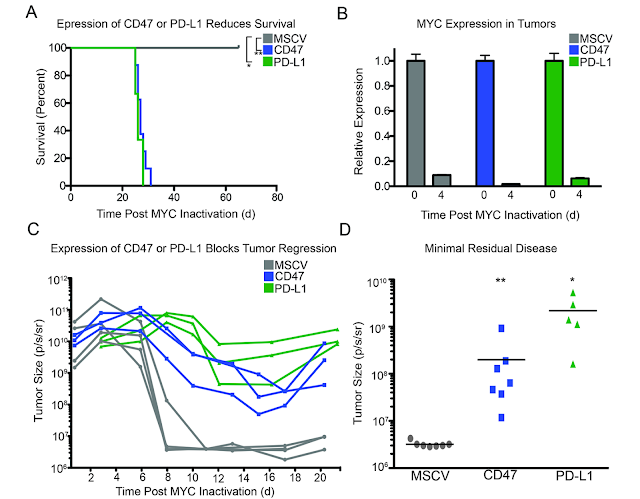
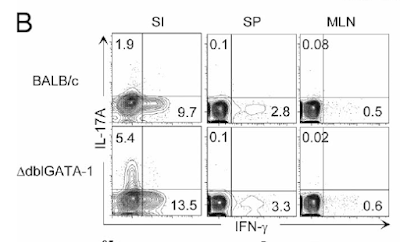
This week I had a hard time to find any good immunology paper that makes me happy to review. Two days ago I read one new paper from immunity with intriguing title about "Organ-Specific Regulatory T Cells" that was so weak (structurally, conceptually and experimentally) that I have no idea how it ended up in that journal. Yesterday I read another new paper from Nature Immunology about dendritic cells (DCs) that had 8 figures in total but only 3 figures were relevant and conclusive, though even that lacked experimental strength. Let me explain my point.
In this paper the authors wanted to find out which type of DCs were involved in oral tolerance (oral tolerance has been used in clinics, for example, to reduce allergy to food products). To do that, the authors compared mice that lacked either monocyte-macrophage–derived DCs [MMDTR mice] or pre-DC–derived classical DCs [zDCDTR mice or CD11cDTR mice as a positive control]. When these mice were fed with OVA protein (to induce oral tolerance) and later challenged with the same antigen in the skin, only mice lacking cDCs showed loss of oral tolerance induction (no reduction in ear swelling or OVA-specific IgG1/2c levels).
Similar results were obtained in upper respiratory airway and lung allergy model (no reduction of eosinophil recruitment in BAL in zDCDTR mice that lacked classical DCs).
Mechanistically, lack of oral tolerance was "associated" with total lack of OVA-specific peripheral [adoptively transferred OT-II] Treg induction in zDCDTR mice (please note that though MMDTR mice showed dramatic reduction of peripheral OVA-Treg generation, it did not affected oral tolerance induction. See Figure 1).
In summary, this study showed that classical DCs were relevant for oral tolerance and antigen-specific peripheral Treg induction, but these two processes were not necessarily connected (at least no experiments were shown to make this connection). So what's the point of showing all these? I don't have an answer to that and probably neither the authors do.
David Usharauli
Similar results were obtained in upper respiratory airway and lung allergy model (no reduction of eosinophil recruitment in BAL in zDCDTR mice that lacked classical DCs).
Mechanistically, lack of oral tolerance was "associated" with total lack of OVA-specific peripheral [adoptively transferred OT-II] Treg induction in zDCDTR mice (please note that though MMDTR mice showed dramatic reduction of peripheral OVA-Treg generation, it did not affected oral tolerance induction. See Figure 1).
In summary, this study showed that classical DCs were relevant for oral tolerance and antigen-specific peripheral Treg induction, but these two processes were not necessarily connected (at least no experiments were shown to make this connection). So what's the point of showing all these? I don't have an answer to that and probably neither the authors do.
David Usharauli