PGE2 is the most abundant eicosanoid lipid in the inflammatory environment and acts via its receptors EP4 and EP2. Earlier this week I wrote about new study that showed the role of PGE2 in suppressing excessive inflammatory response to endogenous microbiota that gained access to internal organs during systemic infection such as sepsis.
This time I am reviewing another new paper about PGE2 from PNAS wherein the authors showed that PGE2 released during the process of cell death modulates its immunogenicity.
Initially, the authors showed that supernatants from cells cultures undergoing various forms of cellular death (freeze-thaw, cisplatin, etoposide or ATP + LPS combination) rather than inducing TNF-α from macrophages it could actually suppress macrophage's response to a canonical inflammatory stimulus such as gram-negative bacterial wall-derived endotoxin, LPS.
Since supernatants treated with DNase I, RNase A, proteinase K or trypsin retained its suppressive activity on the LPS induced production of TNF-α, the authors focus on lipids. Indeed, lipid cellular fraction could reproduce inhibitory effect of the necrotic cell supernatant.
Next, they found that PGE2 was highly enriched in these supernatants and could mediate its suppressive effect.
Synthesis of PGE2 is catalyzed by two cyclooxygenase enzymes, COX-1 and COX-2. Pre-treatment of cells with indomethacin, an inhibitor of COX-1 and COX-2 enzymes, reduced suppressive effect of necrotic cell supernatant.
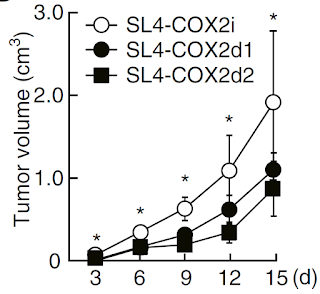
Finally, using CRISPR/Cas9 gene editing technology, the authors constructed COX-2 deficient tumor cell lines and tested their growth pattern in mice. As expected, growth of COX-2 deficient tumor cell lines were delayed in absence of COX-2 enzyme.
In summary, this study showed that cells undergoing sterile cell death (for example, during excessive tumor growth), release suppressive lipid, PGE2, and this mechanism represents one of the natural anti-inflammatory processes that is hijacked by tumors to evade efficient immune detection. Aspirin's beneficial effect as an anti-cancer therapy could be attributed to its effect on PGE2.
David Usharauli




No comments:
Post a Comment