This week Nature Medicine published two papers showing in mouse CAR-T model that cytokine-release syndrome (in both studies) and neurotoxicity (in one study only) were primarily driven by IL-1 and IL-6 cytokines released by monocytes following interaction with infused CAR-T cells.
In the first study led by Michel Sadelain at Sloan Kettering Institute, New York, immunodeficient SCID-beige mice transplanted intra-peritoneally with human B cell tumor cell line and later infused with CD19 CAR-T cells develop cytokine-release syndrome (CRS) that were reversible by anti-IL-6 antibody, injection.
Injection of anti-IL-1 antibody, Anakinra, had similar protective effect against CRS-driven mortality without compromising anti-tumor effectiveness.
However, due to some limitation of their mouse tumor model where CAR-T cells are of human origin and non-T cells such as monocytes are of mouse origin, the authors acknowledged that not all features of CAR-T cell toxicity could be reproduced here since "None of the reported pathologic findings indicative of neuropathology or associated with neurotoxicity (cortical laminar necrosis, hemorrhages, disseminated intravascular coagulation (DIC), gliosis or vasogenic, neurotoxic or interstitial edema) in human patients were observed in any of the mice examined in the present study".
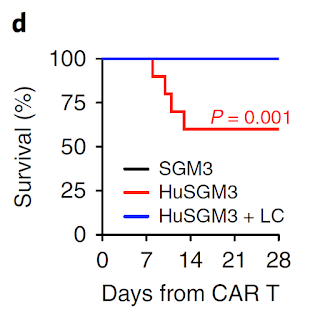
Fortunately, the second study is more extensive and fills much of holes of the first study. Here, the authors led by Attilio Bondanza at San Raffaele Hospital Scientific Institute, Milano, "transplanted human cord blood (CB) hematopoietic stem and progenitor cells (HSPCs) through intrahepatic injection into sublethally irradiated newborn NSG or triple transgenic NSG (SGM3) mice expressing human stem cell factor, granulocytemacrophage colony-stimulating factor (GM-CSF) and IL-3 and initially profiled lymphohematopoietic reconstitution." Basically, this mouse model, referred as newborn humanized SGM3 (nHuSGM3), had both T cell and non-T cell components of hematopoietic system mostly of human origin. In allogeneic tests, human T cells developed in nHuSGM3 mice showed expected functionality.
Staining of cells in nHuSGM3 challenged with tumor and CAR-T cells that induced CRS showed that monocytes were producing IL-1 early on, followed by IL-6.
Depletion of monocytes/macrophages with liposomal clodronate (LC) could rescue mice from CRS.
Importantly, while both anti-IL-1 and anti-IL-6 antibody injection could significantly reduce CRS, only anti-IL-1 antibody were able to reduce neurotoxicity in nHuSGM3 mice. Similar effect were seen with CD44v6 CAR-T cells as well.
In all, these two studies showed that in addition of anti-IL-6 injection, anti-IL-1 antibody therapy could significantly reduce complications of CAR-T cell immunotherapy in humans. They provided a strong evidence to suggest that monocytes were primarily responsible for CRS and neurotoxicity complications of CAR-T cell therapy.
However, it is not clear why are monocytes getting activated after CAR-T cell infusion in the first place. There is interaction, at certain level, between CAR-T cells and monocytes, but is it antigen-specific via anti-CD19 CAR-T or endogenous TCR, or is it non-specific, is unknown presently.
posted by David Usharauli





No comments:
Post a Comment