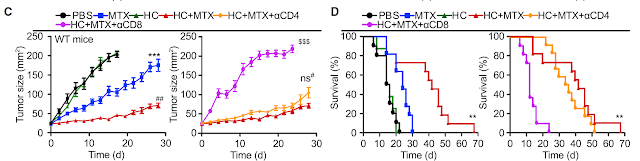
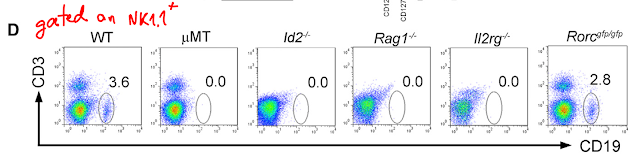
This week journal PNAS published an interesting study on Foxp3+ regulatory T cells (Tregs). It showed that presence of Tregs within secondary lymphoid organs (in lymph nodes) was sufficient to prevent peripheral tissue immunopathology.
As a starting point for this study, the authors showed that mice with Tregs-specific deficiency of kruppel-like factor 2 (KLF2) develop non-fatal peripheral tissue immunopathology, even though in vitro such Tregs [Foxp3-cre; Klf2fl/fl] displayed normal suppressive activity).
Interestingly, in vivo adoptive transfer model, KLF2-KO Tregs also failed to prevent colitis and weight loss when co-transferred with WT naive T cells [but not with KLF2-KO naive T cells].
These results suggested that Tregs re-circulation between tissue and lymphoid tissues could have been involved. Surprisingly, the authors found no difference for Tregs presence [%-wise, no data about #] between KLF2-KO mice or WT (this is probably why this article ended up in PNAS).
Finally, the authors showed that combined deficiency of KLF2 and CCR7 in Tregs accelerated tissue immunopathology [by preventing Tregs access to lymph nodes].
In summary, the authors speculated that Tregs access to lymphoid tissue played a crucial role in preventing tissue immunopathology.
David Usharauli
Interestingly, in vivo adoptive transfer model, KLF2-KO Tregs also failed to prevent colitis and weight loss when co-transferred with WT naive T cells [but not with KLF2-KO naive T cells].
These results suggested that Tregs re-circulation between tissue and lymphoid tissues could have been involved. Surprisingly, the authors found no difference for Tregs presence [%-wise, no data about #] between KLF2-KO mice or WT (this is probably why this article ended up in PNAS).
Finally, the authors showed that combined deficiency of KLF2 and CCR7 in Tregs accelerated tissue immunopathology [by preventing Tregs access to lymph nodes].
In summary, the authors speculated that Tregs access to lymphoid tissue played a crucial role in preventing tissue immunopathology.
David Usharauli