Inflammasome super-complex represents an ancient innate detection and defense mechanism against exogenous as well as endogenous irritants and toxins. Spontaneous inflammasome activity causes several known human immune-pathologies, like Muckle-Well and familial cold auto-inflammatory syndromes.
New paper from Nature Medicine provided evidence showing that alternative energy source, β-hydroxybutyrate, elevated during fasting, calorie-restiction or high-intensity exercise, suppresses NALP3-mediated inflammasome activity.
Initially, the authors led by Prof. Vishwa Deep Dixit at the Yale School of Medicine, showed that β-hydroxybutyrate, but not another ketone body acetoacetate or short-chain fatty acids, butyrate or acetate, could inhibit NALP3-mediated inflammasome activity (AIM2- or NLRC4-mediated inflammasome activities were unaffected).
Suppression of NALP3-mediated inflammasome activity by β-hydroxybutyrate was independent of autophagy and required neither G protein-coupled receptor GPR109a signaling via β-hydroxybutyrate nor histone deacetylase inhibition by β-hydroxybutyrate.
The authors found that β-hydroxybutyrate suppressed NALP3-mediated inflammasome activity through its inhibition (by unknown mechanism) of potassium, K+ cation efflux from cytoplasm.
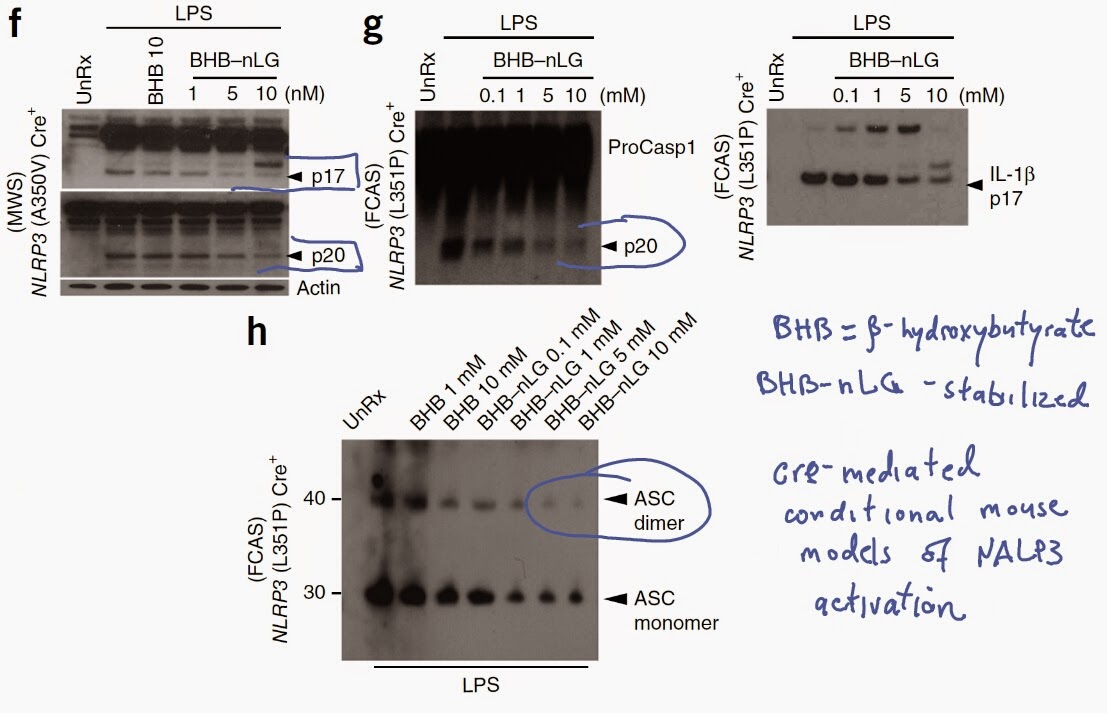
Finally, using cre-mediated mouse conditional models of human Muckle-Well and familial cold auto-inflammatory syndromes, the authors showed that stabilized form of β-hydroxybutyrate could suppress spontaneous NALP3-mediated inflammasome activity and ASC oligomerization characteristics to these gain-of-function NALP3 mutations.
In summary, these results indicate complex interplay between energy metabolism and inflammation. In condition of glucose deficiency, elevated level of β-hydroxybutyrate acts as a new source of ATP in brain and heart and at the same time inhibits NALP3-mediated inflammasome activity.
Leave your comments below and let me know what do you think about this paper.
David Usharauli
Initially, the authors led by Prof. Vishwa Deep Dixit at the Yale School of Medicine, showed that β-hydroxybutyrate, but not another ketone body acetoacetate or short-chain fatty acids, butyrate or acetate, could inhibit NALP3-mediated inflammasome activity (AIM2- or NLRC4-mediated inflammasome activities were unaffected).
The authors found that β-hydroxybutyrate suppressed NALP3-mediated inflammasome activity through its inhibition (by unknown mechanism) of potassium, K+ cation efflux from cytoplasm.
Finally, using cre-mediated mouse conditional models of human Muckle-Well and familial cold auto-inflammatory syndromes, the authors showed that stabilized form of β-hydroxybutyrate could suppress spontaneous NALP3-mediated inflammasome activity and ASC oligomerization characteristics to these gain-of-function NALP3 mutations.
In summary, these results indicate complex interplay between energy metabolism and inflammation. In condition of glucose deficiency, elevated level of β-hydroxybutyrate acts as a new source of ATP in brain and heart and at the same time inhibits NALP3-mediated inflammasome activity.
Leave your comments below and let me know what do you think about this paper.
David Usharauli



No comments:
Post a Comment