Acetyl-CoA plays a critical role in cell metabolism. In normoxic condition, acetyl-CoA is derived from pyruvate. However, in hypoxic condition as in many cancer tissue experience, pyruvate is preferentially converted into lactate. So, how cancer cells generate sufficient level of acetyl-CoA to fuel the growth?
Study in journal Cell suggests that the source of acetyl-CoA in cancer cells is a little known metabolite acetate.
Initially, using hepatocyte cell line, HepG2, and siRNA approach, the authors, led by Drs. Steven McKnight and Benjamin Tu at UT Southwestern Medical Center, Dallas, found that out 3 iso-enzymes capable of acetate conversion into acetyl-CoA and its incorporation into lipid or protein synthesis, iso-form 2 (acetyl-CoA synthetase enzyme 2) was mostly responsible for this action in an vitro assay.
Similar results were obtained when cells harvested from mice deficient in both or one allele of iso-form 2 were tested.
Interestingly, examination of several tumor cell lines revealed that inhibition of iso-form 2 had a major impact on acetate incorporation into lipids or proteins.
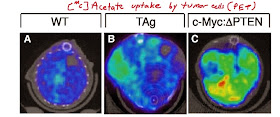
PET image analysis confirmed that tumors could efficiently incorporate acetate.
More importantly, using inducible or spontaneous liver tumor models, the authors observed that iso-form 2-deficient mice showed diminished tumor burden (both in size and quantity).
Finally, analysis of human tissue samples showed high level of expression of iso-form 2 in cancer tissue compared to normal cells.
In summary, the results of this study, and another study on human glioblastoma, led by Elizabeth Maher and Robert Bachoo (from the same research center), suggest new treatment path for cancer therapy.
David Usharauli





No comments:
Post a Comment