This week several science news outlets spotlighted new study from Nature Immunology showing anti-tumor effect of Cish deficient NK cells. This study showed that Cish deficient NK cells are hyper-responsive to its canonical cytokine IL-15 and show improved control of experimental tumors.
I analysed this study to determine if the buzz was deserving. In my view this study is in fact two independent [and not connected] studies put together artificially. The finding that Cish deficient NK cells have superior anti-tumor behavior is based on non-physiological experimental model.
First part of this study deals with cytokine sensitivity of Cish deficient NK cells. This is exclusively in vitro experiments. It does show that Cish deficient NK cells are hyper-responsive to NK cell canonical cytokines such as IL-15, or IL-12/IL-18 combo [and other activatory receptors].
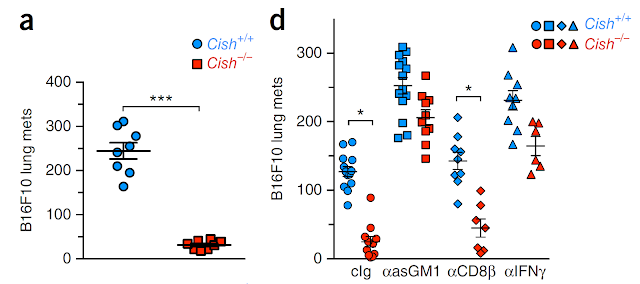
Second part of this study, however, is completely dissociated with IL-15 story and jumps directly to tumor protection experiments with WT or Cish deficient NK cells. These experiments showed that Cish deficient mice are resistant to exogenously injected tumor cells and this protection depended on asiolo-GM1+ cells and IFN-γ.
Finally, the authors showed that adoptive transfer of Cish deficient NK cells into NK-deficient hosts (Ncr1Mcl1Δ/Δ mice) also provided protection against i.v. injected melanoma cell metastasis.
In summary, this study proposed that Cish functions as a checkpoint inhibitor for NK cells.
My view:
(A) It is not clear whether Cish deficient NK cells alone is sufficient for anti-tumor effect [it is independent of CD8 T cells, but CD4 T cells involvement was not tested].
(B) It is not clear what role cytokines such as IL-15 or IL-12/18 play in vivo in Cish deficient mice.
(C) One of the major differences between NK and T cells is that NK cell effector functions are not regulated in an antigen-specific manner but rather by sensing signaling balance between activatory and inhibitory membrane receptors [functions like a rheostat].
During ontogeny individual NK cell undergoes its own "adjustment" to its environment and can even "tolerate" naturally arising tumor cells. This is why experimental tumor models when tumors are injected exogenously, and appear in the body out of the blue so to speak, do not recapitulate natural interaction with NK cells and easily could produce biased, non-physiological response from first-time encountering NK cells. The more physiological tumor models will be to use spontaneously arising mouse tumor models.
David Usharauli
I analysed this study to determine if the buzz was deserving. In my view this study is in fact two independent [and not connected] studies put together artificially. The finding that Cish deficient NK cells have superior anti-tumor behavior is based on non-physiological experimental model.
First part of this study deals with cytokine sensitivity of Cish deficient NK cells. This is exclusively in vitro experiments. It does show that Cish deficient NK cells are hyper-responsive to NK cell canonical cytokines such as IL-15, or IL-12/IL-18 combo [and other activatory receptors].
Second part of this study, however, is completely dissociated with IL-15 story and jumps directly to tumor protection experiments with WT or Cish deficient NK cells. These experiments showed that Cish deficient mice are resistant to exogenously injected tumor cells and this protection depended on asiolo-GM1+ cells and IFN-γ.
Finally, the authors showed that adoptive transfer of Cish deficient NK cells into NK-deficient hosts (Ncr1Mcl1Δ/Δ mice) also provided protection against i.v. injected melanoma cell metastasis.
In summary, this study proposed that Cish functions as a checkpoint inhibitor for NK cells.
My view:
(A) It is not clear whether Cish deficient NK cells alone is sufficient for anti-tumor effect [it is independent of CD8 T cells, but CD4 T cells involvement was not tested].
(B) It is not clear what role cytokines such as IL-15 or IL-12/18 play in vivo in Cish deficient mice.
(C) One of the major differences between NK and T cells is that NK cell effector functions are not regulated in an antigen-specific manner but rather by sensing signaling balance between activatory and inhibitory membrane receptors [functions like a rheostat].
During ontogeny individual NK cell undergoes its own "adjustment" to its environment and can even "tolerate" naturally arising tumor cells. This is why experimental tumor models when tumors are injected exogenously, and appear in the body out of the blue so to speak, do not recapitulate natural interaction with NK cells and easily could produce biased, non-physiological response from first-time encountering NK cells. The more physiological tumor models will be to use spontaneously arising mouse tumor models.
David Usharauli


betmatik
ReplyDeletekralbet
betpark
tipobet
slot siteleri
kibris bahis siteleri
poker siteleri
bonus veren siteler
mobil ödeme bahis
ZDKL
dijital kartvizit
ReplyDeletereferans kimliği nedir
binance referans kodu
referans kimliği nedir
bitcoin nasıl alınır
resimli magnet
1F6
adana
ReplyDeleteşişli
sakarya
elazığ
kadıköy
FRT